CPD frameworks
These frameworks, associated with all articles, prompt drafting of personal learning, reflection and planning.
Save your reflective note into your device or cloud
Fillable PDF frameworks
Adobe reader needed for tablets
Word frameworks - for reflective practice
Click to download
Reflection on a journal article
Stages of reflection on a situation
Reflection of a team, practice or group
External reference on reflection
Reflective practice in health care and how to reflect effectively
Koshy K, Limb C et al. International Journal of Surgical Oncology. 2017 2:e20
Jalal M, Hopper AD. Diagnosis and management of oesophageal cancer. Feb 2018;262(1812):21-25
Diagnosis and management of oesophageal cancer
22 Feb 2018
AUTHORS
Dr Mustafa Jalal MRCP, Clinical Fellow in Gastroenterology
Dr Andrew D Hopper MD FRCP, Gastroenterology Consultant, Department of Gastroenterology, Royal Hallamshire Hospital, Sheffield, UK
Competing interests: None
Article
Abstract
Oesophageal cancer rates are continuing to increase. Oesophageal cancer is the thirteenth most common cancer in the UK, however, its outcome remains poor making it the fifth most common cause of cancer deaths. There are two main types; oesophageal squamous cell carcinoma (OSCC) and oesophageal adenocarcinoma (OAC). They have different pathogenesis but similar presentation. Both carry a very poor five-year survival of 17% compared with more common cancers such as colorectal (60%), prostate (88%) and breast cancer (86%). Oesophageal cancer is twice as common in men than women with peak incidence at presentation in the 65-75 age group. Smoking is a major risk factor for oesophageal cancer and is linked to an estimated two-thirds of cases in the UK. Other risk factors include excess alcohol intake, chewing betel leaf, GORD, obesity and Barrett’s oesophagus. Oesophageal cancer commonly presents with dysphagia or odynophagia and can be associated with weight loss and vomiting. Referral for urgent endoscopy should always be considered in the presence of dysphagia regardless of previous history or medication. Dysphagia, weight loss and age are strong positive predictors of cancer. NICE recommends urgent referral (within 2 weeks) for direct access for upper GI endoscopy in patients with dysphagia and those aged 55 years or over with weight loss and any of the following: upper abdominal pain, reflux, or dyspepsia. If the patient has no distant metastases or local invasion, a clinical assessment for curative treatment including surgery is carried out. Tumours that show local invasion or distant metastases are not amenable to curative treatment. The majority of patients with oesophageal cancer have incurable metastases at diagnosis.
Oesophageal cancer rates are continuing to increase with 7,500 new cases diagnosed in the UK in 2016 compared with 7,000 in 2013. Oesophageal cancer is the thirteenth most common cancer in the UK,1 however, its outcome remains poor making it the fifth most common cause of cancer deaths.2
Early diagnosis improves survival rates. Both the National Institute for Health and Care Excellence (NICE)3 and European Society of Medical Oncology (ESMO)4 have published updated guidance which aims to improve outcomes in this potentially curable cancer.
The two main types of oesophageal cancer, oesophageal squamous cell carcinoma (OSCC) and oesophageal adenocarcinoma (OAC), have different pathogenesis but similar presentation. Both carry a very poor five-year survival of 17% compared with the more common cancers such as colorectal (60%), prostate (88%) and breast cancer (86%).2
The UK has the highest incidence of OAC in Europe in females and the second highest in males.5 OAC is the more common form of oesophageal cancer seen in the UK and other developed countries, whereas OSCC remains more common globally.6
Oesophageal cancer is twice as common in men than women and the vast majority of patients are over the age of 50 at presentation with peak incidence in the 65-75 age group.5
Risk factors for oesophageal cancer are listed in box 1.
Presentation
Patients commonly present with dysphagia or odynophagia which may be accompanied by weight loss and vomiting. Although a variety of other conditions may cause dysphagia, see table 1, referral for urgent endoscopy should always be considered in the presence of dysphagia regardless of previous history or medication. Dysphagia, weight loss and age are strong positive predictors for cancer. In a study on symptom referral for rapid access endoscopy, 92% of patients with malignancy had either dysphagia, weight loss or were over the age of 55 with other alarm symptoms.14 Because of its importance as a predictor for cancer, any subjective history of weight loss in the absence of any known illness should be considered.15,16
Advanced tumours can present without dysphagic symptoms due to the elastic nature of the oesophagus. Patients with advanced disease may present with anaemia and haematemesis, resulting from bleeding lesions, hoarse voice caused by early mediastinal invasion or weight loss which may indicate metastatic spread.
At risk or alarm symptoms for oesophago-gastric cancer that should prompt endoscopy referral have been highlighted in the NICE guidelines for referral for suspected cancer17 and ESMO4 guidelines and also previously published guidelines by the British surgical and gastroenterological societies.18
The NICE recommendations for endoscopy referral to assess for suspected upper gastrointestinal (GI) and oesophageal cancer are shown in table 2.17 The recommendations from NICE differ slightly from those in other earlier guidelines regarding who to refer urgently or to consider for non-urgent endoscopy. The British surgical and gastroenterological societies recommend rapid access endoscopy for all patients over 55 with recent onset dyspepsia regardless of a response to treatment or all patients with alarm symptoms irrespective of age.18 Guidance from ESMO is similar to that from NICE and recommends an upper GI endoscopy for all patients with new dysphagia, GI bleeding, recurrent aspiration or nausea, weight loss and/or loss of appetite.4
‘Dysphagia, weight loss and age are strong positive predictors for oesophageal cancer’
As the NICE guidance is the most recent it should probably be used as initial guidance for the urgency of referral for direct endoscopy. However, patients over 55 with dyspepsia should be thoroughly reviewed to assess for a complete response to treatment.
Non-urgent referral for endoscopy is advisable when there is any clinical suspicion, persisting unexplained upper GI symptoms, or proton pump inhibitor (PPI) treatment is required long term (> 6 weeks).
Diagnosis
Patients with dysphagia should undergo upper GI endoscopy with biopsy to confirm a diagnosis of oesophageal cancer. Lesional biopsy with histological interpretation is used to identify the cancer subtype and to exclude other potential causes such as severe GORD and ulceration, see table 1. If histology is benign but endoscopic appearances were suspicious of cancer, gastroscopy should be repeated. In severe reflux, gastroscopy with biopsies is repeated after 6 weeks of anti-acid treatment to ensure healing and exclude underlying cancer or Barrett’s oesophagus or earlier if symptoms are progressive on PPI. Failure to diagnose oesophageal and gastric cancer at initial endoscopy is consistently around 10%. Hence, patients with unexplained symptoms should be considered for a second gastroscopy.19, 20
PPIs may mask endoscopic findings and alter the appearance of malignant ulcers. Initial gastroscopy should follow a break in PPI therapy, usually of two weeks although there is no evidence on the best timing. If the patient is unable or unwilling to tolerate gastroscopy barium studies can be carried out.21
This approach may detect a stricture or mucosal ulceration. However, it does not enable histological sampling to differentiate between malignant and benign ulceration so diagnosis could be delayed.
Disease staging
If gastroscopy reveals a lesion suspicious of oesophageal cancer, the patient is warned and referred to a specialist upper GI surgery unit.
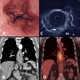
The staging process enables patients and their doctors to choose appropriate treatments and avoid unnecessary surgery in those with advanced or incurable disease, see figure 1. Oesophageal cancer staging employs the Tumour, Nodal, Metastases (TNM) classification system.22
Computed tomography (CT) of the neck, chest, abdomen and pelvis (whole body) is carried out initially to detect incurable disease.3,4 CT has a 90% sensitivity for detecting distant metastasis > 1 cm and 85-100% sensitivity for detecting significant local invasion into adjacent mediastinal organs.23-25 If the patient has no distant metastases or local invasion, a clinical assessment for suitability for curative treatment including surgery is carried out. This will often include a formal objective cardiorespiratory assessment including exercise tolerance26 or complex cardiopulmonary exercise testing.27
Patients being considered for radical, curative treatment undergo complete TNM staging. Positron emission tomography (PET-CT) using F-18 fluorodeoxyglucose is recommended in all patients to detect distant lymph node or metastatic disease.3 Around 5-15% of patients with oesophageal cancer who are initially thought to be suitable for surgery are precluded from oesophagectomy after PET-CT staging.28 Endoscopic ultrasound (EUS) can provide further assessment of, and possible tumour invasion into, nearby organs (such as the aorta) and sample suspicious lymph nodes but given its limited effect on management it is now only recommended in specific patients.3,29
Staging laparoscopy is indicated where the tumour involves the lower oesophagus and upper stomach and CT demonstrates potentially operable disease. Laparoscopy is able to detect peritoneal and metastatic disease < 5 mm in diameter, and enables peritoneal cytology and biopsies to be obtained from suspicious lesions. Staging laparoscopy alters initial treatment decisions for invasive surgery in up to 28% of patients with gastric cancer following CT.30 The stage of a cancer is closely related to prognosis emphasising the importance of early diagnosis.31
There is a high rate of synchronous second squamous lung and throat cancer in patients with OSCC due to chronic smoking and alcohol consumption therefore there is a low threshold for bronchoscopy and ear, nose and throat specialist review to exclude synchronous second cancer.20
‘There is a high rate of synchronous second squamous lung and throat cancer in patients with oesophageal squamous cell carcinoma’
Patients should be supported by cancer nurse specialists and specialist dietitians during the staging process. The complex staging process is key to decision-making regarding suitability for curative surgery and treatment. If surgery is performed unnecessarily in patients with advanced disease the recovery from surgery may have a significant impact on the patient’s quality of life for their remaining life expectancy.
Management
The specialist upper GI multidisciplinary team (MDT) involves consultant surgeons, radiologists, pathologists, oncologists, gastroenterologists, palliative care physicians as well as cancer nurse specialists and specialist dietitians. The patients’ investigation results are considered alongside their fitness for surgery and/or chemotherapy at the MDT meeting. The final decision should be made together with the patient after the recommended treatment options have been carefully explained. Tumours that show local invasion (T4) or distant metastases (M1) are not amenable to curative treatment.
Curative treatment
Advances in endoscopic imaging now result in detection of early, non-ulcerating carcinomas at screening and before dysphagia develops. In small nodular lesions < 2 cm, endoscopic mucosal resection (EMR) may be considered to stage and treat early cancers and differentiate between high grade dysplasia, T1a (tumour invades lamina propria or muscularis mucosae) and T1b (tumour invades submucosa) lesions.4,32 Endoscopic removal may be complete and considered curative in T1a given the low incidence of lymph node metastases in this group (< 5%), and avoids surgery. Although T1b OAC can be fully removed with EMR, patients should be offered radical resection if they are fit enough to have surgery due to the higher risk of lymph node metastases up to 20%.12 Patients deemed medically fit with localised, non-metastatic OAC should be offered surgical resection. This is recommended with the addition of chemotherapy before or before and after surgery or chemoradiotherapy only before surgery.3
Curative chemoradiotherapy may be an option for localised OSCC especially if it is affecting the upper oesophagus. Although surgery appears to be a better option in comparison,33 some studies have shown equivalent two-year survival to surgery in this group, therefore chemoradiotherapy remains a recommended first-line option for OSCC.18,34 The benefits, risks and treatment consequences of each option need to be fully discussed with the patient.
Oesophagectomy is highly invasive surgery and is performed by either an abdominal incision and trans-hiatal approach (to mobilise the stomach and a subsequent neck incision to pull up the stomach into the mediastinum
and remove the oesophagus) or as a trans-thoracic Ivor-Lewis oesophago-gastrectomy which involves an abdominal incision and a left-sided thoracotomy. The two approaches have similar in hospital mortality and five-year survival rates.
Oesophagectomy is associated with significant morbidity and complications (33%),35 in hospital mortality (1.9%) and 90-day mortality rate of (3.2%)36 with reduction in long-term quality of life.37 Surgery should be performed in centres with higher case volumes to achieve better results, and the outcomes of surgery are subject to significant national audit data.38,39
Multiple meta-analyses have shown the benefits of preoperative (neoadjuvant) chemotherapy or chemoradiotherapy in patients undergoing surgery.4 A large UK study found that two cycles of neoadjuvant chemotherapy improved survival over two years from 34 to 43% with no additional serious adverse events.
This effect is notable especially for patients with T3 disease (tumour invades adventita) or the presence of cancer in lymph nodes and is therefore used in most UK centres.40-42
Palliative treatment
The majority of patients presenting with oesophageal cancer have incurable metastases at diagnosis. A palliative treatment plan should be considered by the MDT, taking into account performance status and the views of the patient. Early direct involvement of the palliative care team, the cancer nurse specialists and dietitians is vital.
Palliative combination chemotherapy is an option in advanced oesophageal cancer. Studies have demonstrated a response to palliative chemotherapy in 37-48% of patients. Mean survival is 8-13 months with better outcomes in patients with OSCC.43,44 In patients with advanced OAC involving the upper stomach, endoscopic biopsies are assessed for HER-2 immunopositivity. The addition of trastuzumab can result in a significant improvement in response rate and median overall survival (13.8 vs 11.1 months) in those with HER-2 receptive tumours.45,46
Self-expanding metal stents (SEMS) can be used to relieve dysphagia and aid nutrition. They can be placed endoscopically or radiologically bringing relief in a single procedure.18 When compared with other methods to help swallowing, such as endoscopy with argon photocoagulation debulking, SEMS have similar outcomes on quality of life, but debulking requires multiple procedures so is avoided in those patients with limited life expectancy.47 Complications include stent migration, pain for up to 10 days, blockage and stent overgrowth by tumour requiring further stent or endoscopy in a third of cases.48
External beam radiotherapy should be considered after SEMS insertion for long-term disease control.3
Support and follow-up
Nutritional assessment and review by a dietitian should be offered before, during and after radical or palliative treatment.3 Input from dietitians and cancer nurse specialists has been shown to contribute to a faster recovery from treatment and improved quality of life.49 Regular review of patients following therapy is essential to manage post-treatment side effects such as dysphagia and post-surgical diarrhoea and pain. Nutrition and psychological support should be included in these visits.4 Follow-up and radiological surveillance should not be offered solely for the detection of recurrence.3
The cancer nurse specialist acts as the patient’s advocate and can ensure close liaison with primary and secondary care and help avoid readmission for relief of pain, nutrition and dysphagia.50
Conclusion
Oesophageal cancer still has one of the lowest cancer survival outcomes in the UK and a low threshold for early endoscopy for dysphagic symptoms is recommended. Although cancer registries across Europe report gradual improvements in five-year survival rates, they are still generally poor and varied.
The observed trends reflect the variations in alcohol consumption, tobacco smoking and obesity across different countries.51 Preventative strategies including smoking cessation and weight reduction will improve rates of oesophageal cancer.
Advances in minimal access surgery and developments in endoscopy are encouraging.52
REFERENCES
1 Cancer registration statistics, England: first release, 2016. Office for National Statistics; 2018. Last accessed 17 February 2018
2 Cancer survival in England: adult, stage at diagnosis and childhood – patients followed up to 2016. Office for National Statistics; 2017 . Last Accessed 17 February 2018
3 National Institute for Health and Care Excellence. NG83. Oesophago-gastric cancer: assessment and management in adults. NICE. London. 2018
4 Lordick F, Mariette C, Haustermans K et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27(suppl 5):v50-v7
5 Cancer Research UK. Oesophageal cancer statistics. Last Accessed 17 February 2018
6 Chung CS, Lee YC, Wang CP et al. Secondary prevention of esophageal squamous cell carcinoma in areas where smoking, alcohol, and betel quid chewing are prevalent. J Formos Med Assoc 2010;109:408-21
7 Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med 1999;340:825-31
8 Steffen A, Huerta JM, Weiderpass E et al. General and abdominal obesity and risk of esophageal and gastric adenocarcinoma in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2015;137:646-57
9 Bhat S, Coleman HG, Yousef F et al. Risk of malignant progression in Barrett's esophagus patients: results from a large population-based study. J Natl Cancer Inst 2011;103:1049-57
10 Hvid-Jensen F, Pedersen L, Drewes AM et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375-83
11 Theron BT, Padmanabhan H, Aladin H et al. The risk of oesophageal adenocarcinoma in a prospectively recruited Barrett's oesophagus cohort. United European Gastroenterol J 2016;4(6):754-61
12 Fitzgerald RC, di Pietro M, Ragunath K et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7-42
13 Hopper AD. Improving the diagnosis and management of GORD in adults. Practitioner 2015;259:27-32
14 Kapoor N, Bassi A, Sturgess R, Bodger K. Predictive value of alarm features in a rapid access upper gastrointestinal cancer service. Gut 2005;54:40-5
15 Wong CJ. Involuntary weight loss. Med Clin North Am 2014;98:625-43
16 Gaddey HL, Holder K. Unintentional weight loss in older adults. Am Fam Physician 2014;89:718-22
17 National Institute for Health and Care Excellence. NG12. Referral guidelines for suspected cancer. NICE. London. 2015. www.nice.org.uk/guidance/ng12
18 Allum WH, Blazeby JM, Griffin SM et al. Guidelines for the management of oesophageal and gastric cancer. Gut 2011;60:1449-72
19 Yalamarthi S, Witherspoon P, McCole D, Auld CD. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy 2004;36:874-9
20 Bramble MG, Suvakovic Z, Hungin AP. Detection of upper gastrointestinal cancer in patients taking antisecretory therapy prior to gastroscopy. Gut 2000;46:464-7
21 Levine MS, Chu P, Furth EE et al. Carcinoma of the esophagus and esophagogastric junction: sensitivity of radiographic diagnosis. AJR Am J Roentgenol 1997;168:1423-6
22 Rice TW, Ishwaran H, Blackstone EH. Oesophageal cancer: location, location, location. Eur J Cardiothorac Surg 2015;48:194-5
23 Kuszyk BS, Bluemke DA, Urban BA et al. Portal-phase contrast-enhanced helical CT for the detection of malignant hepatic tumors: sensitivity based on comparison with intraoperative and pathologic findings. AJR Am J Roentgenol 1996;166:91-5
24 Picus D, Balfe DM, Koehler RE et al. Computed tomography in the staging of esophageal carcinoma. Radiology 1983;146:433-8
25 Umeoka S, Koyama T, Watanabe G et al. Preoperative local staging of esophageal carcinoma using dual-phase contrast-enhanced imaging with multi-detector row computed tomography: value of the arterial phase images. J Comput Assist Tomogr 2010;34:406-12
26 Murray P, Whiting P, Hutchinson SP et al. Preoperative shuttle walking testing and outcome after oesophagogastrectomy. Br J Anaesth 2007;99:809-11
27 Feeney C, Hussey J, Carey M, Reynolds JV. Assessment of physical fitness for esophageal surgery, and targeting interventions to optimize outcomes. Dis Esophagus 2010;23:529-39
28 Meyers BF, Downey RJ, Decker PA et al. The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the American College of Surgeons Oncology Group Z0060 trial. J Thorac Cardiovasc Surg 2007;133:738-45
29 Pech O, Gunter E, Dusemund F et al. Accuracy of endoscopic ultrasound in preoperative staging of esophageal cancer: results from a referral center for early esophageal cancer. Endoscopy 2010;42:456-61
30 de Graaf GW, Ayantunde AA, Parsons SL et al. The role of staging laparoscopy in oesophagogastric cancers. Eur J Surg Oncol 2007;33:988-92
31 Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol 2010;17:3077-79
32 Cao Y, Liao C, Tan A et al. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy 2009;41(9):751-7
33 Stahl M, Stuschke M, Lehmann N et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310-7
34 Bedenne L, Michel P, Bouche O et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007;25:1160-08
35 National Oesophago-gastric Cancer Audit - 2014, Annual Report. 27 Nov 2014 Last accessed 12 Feb 2018
36 National Oesophago-Gastric Cancer Audit - 2016, Annual Report. 8 Sep 2016 Last accessed 12 February 2018
37 Djarv T, Lagergren J, Blazeby JM, Lagergren P. Long-term health-related quality of life following surgery for oesophageal cancer. Br J Surg 2008;95:1121-26
38 National Oesophago-gastric Cancer Audit - 2013, Annual report: June 26, 2013.
39 Bachmann MO, Alderson D, Edwards D et al. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg 2002;89:914-22
40 Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359:1727-33
41 Cunningham D, Allum WH, Stenning SP et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20
42 Sjoquist KM, Burmeister BH, Smithers BM et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92
43 Van Cutsem E, Moiseyenko VM, Tjulandin S et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-97
44 Cunningham D, Starling N, Rao S et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46
45 Bang YJ, Van Cutsem E, Feyereislova A et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97
46 Rivera F, Carrato A, Gravalos C et al. Recommendations on current approach to gastric cancer. Clin Transl Oncol 2009;11:518-25
47 Diamantis G, Scarpa M, Bocus P et al. Quality of life in patients with esophageal stenting for the palliation of malignant dysphagia. World J Gastroenterol 2011;17:144-50
48 Mangiavillano B, Pagano N, Arena M et al. Role of stenting in gastrointestinal benign and malignant diseases. World J Gastrointest Endosc 2015;7:460-80
49 Isenring EA, Capra S, Bauer JD. Nutrition intervention is beneficial in oncology outpatients receiving radiotherapy to the gastrointestinal or head and neck area. Br J Cancer 2004;91:447-52
50 Leary A, Crouch H, Lezard A et al. Dimensions of clinical nurse specialist work in the UK. Nurs Stand 2008;23:40-4
51 Castro C, Bosetti C, Malvezzi M et al. Patterns and trends in esophageal cancer mortality and incidence in Europe (1980-2011) and predictions to 2015. Ann Oncol 2014;25:283-90
52 Safranek PM, Cubitt J, Booth MI, Dehn TC. Review of open and minimal access approaches to oesophagectomy for cancer. Br J Surg 2010;97:1845-53
53 Abdel Jalil AA, Katzka DA, Castell DO. Approach to the patient with dysphagia. Am J Med 2015;128:1138 e17-23
ARTICLE IN PDF
PRESET SEARCHES
PubMed
Outcomes for oesophageal cancer review
US NCI
Oesophageal cancer(PDQ summaries)
NICE evidence search

 = Registered users
= Registered users = Paid-up subscribers
= Paid-up subscribers